Pyrolysis
Welcome to the world of Pyrolysis! This will guide you through the pyrolysis process, its applications, and its potential as a renewable energy source. Get ready to embark on your pyrolysis learning journey!
Workshop Plan
Workshop Presentation
Welcome Abroad
Welcome to the Pyrolysis Topic
Introductory Video
Discover insightful explanations and demonstrations in the following video.
Topic Orientation
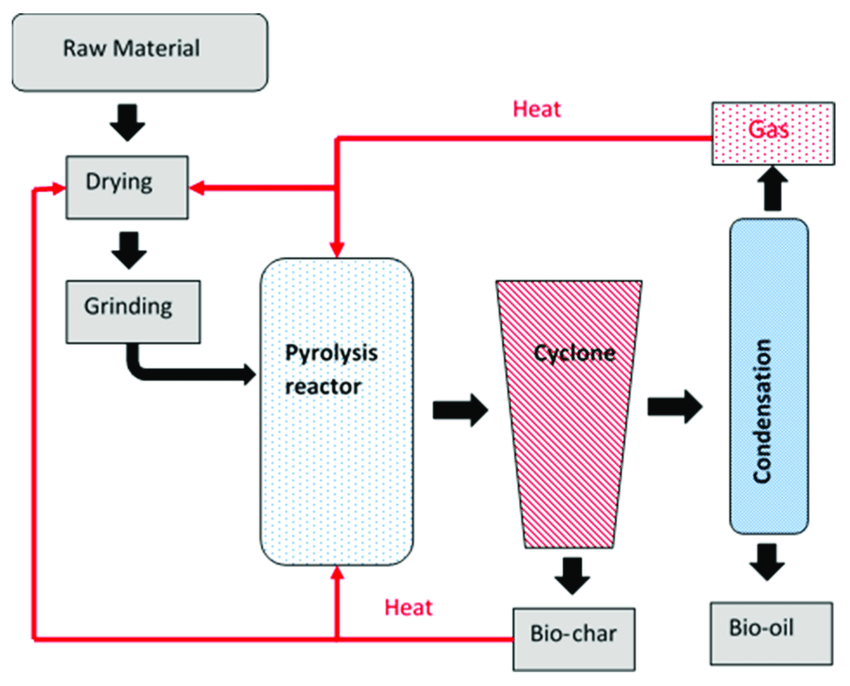
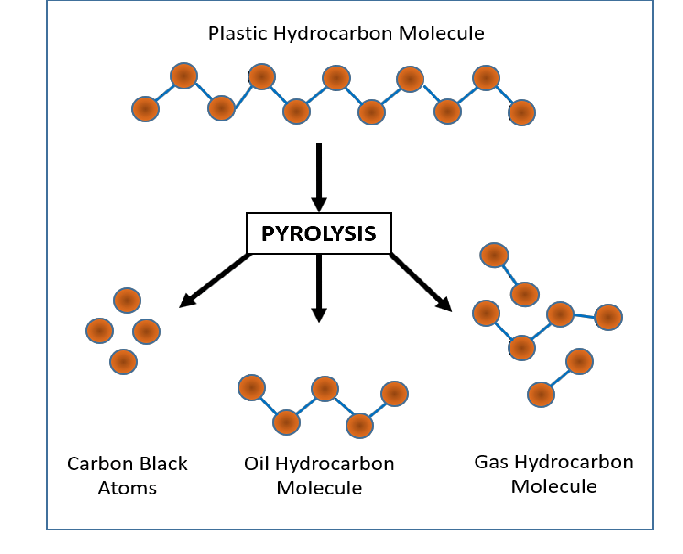
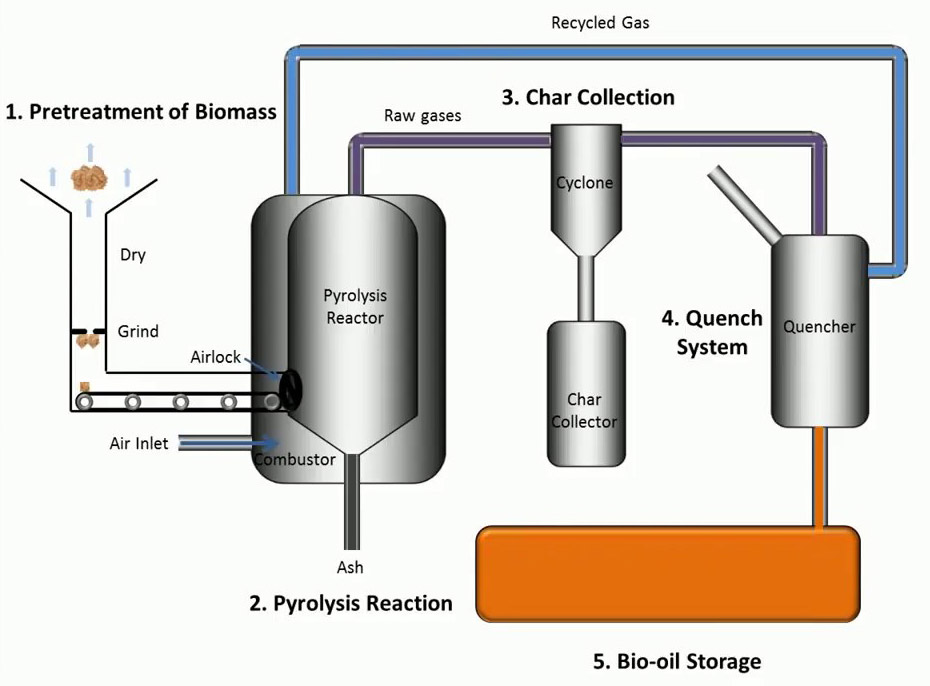
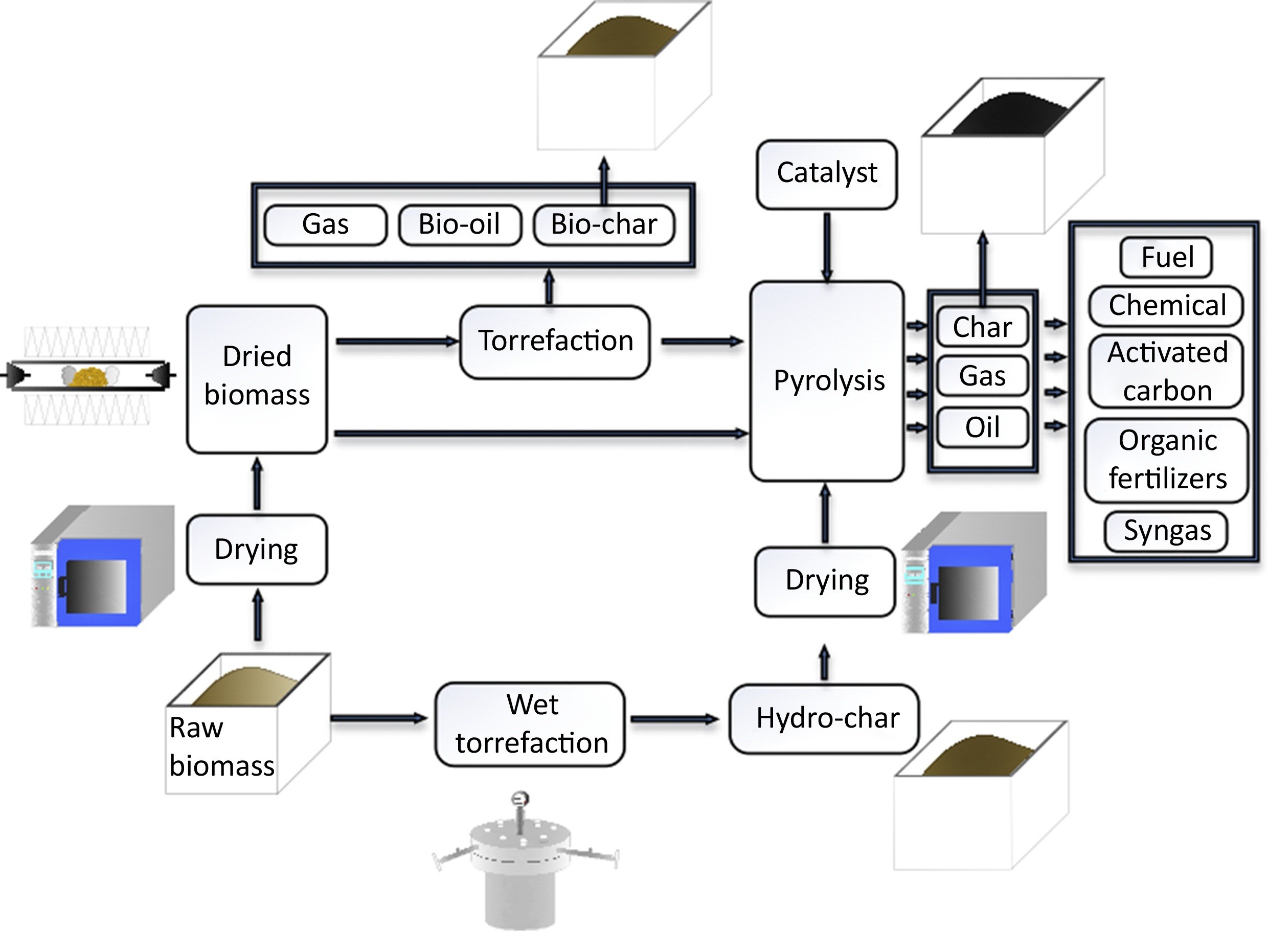
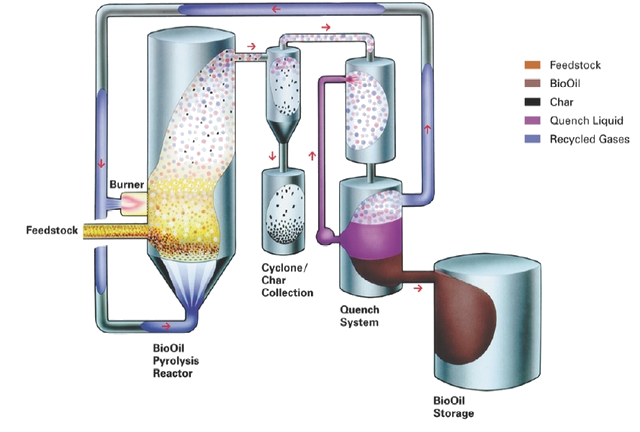
Insightful Visuals
Informative Visuals of Pyrolysis Reaction.
Case Studies
Dow and Mura Technology’s Pyrolysis Plant in Böhlen, Germany
- Dow and Mura Technology have partnered to build a pyrolysis plant in Böhlen, Germany.
- The plant will convert mixed plastic waste into hydrocarbon liquids, which will be used by Dow to produce new plastics.
- The facility will divert 120,000 metric tons of waste per year from incinerators and is expected to be the largest of its kind in Europe.
- The partners plan to build additional facilities at Dow sites in Europe and the US, with a total capacity of 600,000 metric tons per year.

Nexus Circular’s Pyrolysis Process
- Nexus Circular is a pyrolysis firm that focuses on dealing with heterogeneous inputs.
- They aim to convert mixed plastic waste into uncontaminated feedstock for the production of new polymers.
- The company emphasizes the challenge of dealing with the variability of feedstock and the need to select the appropriate temperature for breaking down different polymers.

Brightmark Energy Plant, Indiana
Brightmark Energy operates a pyrolysis plant that aims to recycle plastic waste into fuels such as diesel fuel, naphtha, and wax.

Software Application
Step 1: Reaction Rate (k)

The reaction rate constant (k) is a crucial parameter in pyrolysis kinetics. It depends on various factors, including temperature, feedstock composition, and the presence of a catalyst. Choose values that match your specific pyrolysis conditions.
Step 2: Degree of Conversion (α)

The degree of conversion (α) represents the fraction of reactant that has undergone pyrolysis. It depends on reaction time, temperature, feedstock composition, and the presence of a catalyst.
Step 3: Reaction Orders (n and m)

The reaction orders (n and m) determine how the reactants affect the reaction rate. Choose values for ‘n’ and ‘m’ that correspond to the reactant orders in your pyrolysis kinetics equation.
Step 4: Calculate Output (dα/dt)

Click the button to compute the output (dα/dt), the rate of weight loss at a given time, based on the parameters you’ve selected in the previous steps and the pyrolysis kinetics equation.
Flip The Cards
Matching Words
Match the terms with their definitions!
- is a thermal decomposition process that converts biomass into biochar, syngas, and other useful products.
- is a solid, carbon-rich product of the pyrolysis process that can be used as a soil amendment or for other applications.
- is a mixture of carbon monoxide, hydrogen, and other gases produced during the pyrolysis process, which can be used as a fuel.
- refers to organic matter, such as plants or agricultural waste, that can be used as a feedstock for the pyrolysis process to produce energy.
Good Job !
There are still unanswered questions!
Smooth Quiz
Pyrolysis Quiz
What is the process by which biomass is converted into energy?
What is the primary material used in the production of biomass energy?
Entrepreneurial Spark
Pyrolysis: Business Ideation
Seed Research Paper
Under Development, Stay Tuned!
Hands-On Kit
Experience hands-on learning with the educational practical kit.
Formula Fundamentals
In the field of pyrolysis, several equations are used to describe the different aspects of the process. These equations help in understanding the kinetics, heat transfer, and mass transfer phenomena involved in pyrolysis. Here are some of the main equations used in the study of pyrolysis:
- Kinetic Equations:
- Arrhenius equation: Describes the temperature dependence of the reaction rate in pyrolysis [1].
- Reaction rate equations: Various reaction rate models, such as the first-order, nth-order, or parallel reactions, can be used to describe the conversion of biomass into different products during pyrolysis [1].
- Heat Transfer Equations:
- Fourier's law of heat conduction: Describes the heat transfer through the solid biomass particles during pyrolysis [2].
- Heat transfer coefficient equations: Empirical correlations or models are used to calculate the convective heat transfer coefficient between the solid particles and the surrounding gas [2].
- Mass Transfer Equations:
- Fick's law of diffusion: Describes the mass transfer of volatile species from the solid biomass particles to the gas phase during pyrolysis [2].
- Mass transfer coefficient equations: Empirical correlations or models are used to calculate the convective mass transfer coefficient between the solid particles and the surrounding gas [2].
- Energy Balance Equations:
- Energy balance equation: Accounts for the energy transfer and conversion during pyrolysis, including the heat of reaction, sensible heat, and latent heat.
- Conservation of energy equation: Balances the energy inputs and outputs in the pyrolysis system, including the heat supplied, heat losses, and heat absorbed by the products.
These equations, along with experimental data and other parameters, are used in mathematical models to simulate and predict the behavior of pyrolysis processes.
Learn more:
Supplementary Resources
Here are some credible references discussing the subject of Pyrolysis.
| Reference Title | Source |
|---|---|
| "Pyrolysis" by the U.S. Department of Agriculture (USDA) | USDA Research on Pyrolysis |
| "Pyrolysis of Biomass for Bioenergy" by the National Renewable Energy Laboratory (NREL) | NREL Document on Pyrolysis |
| "Pyrolysis: An Overview" by the International Energy Agency (IEA) | IEA Article on Pyrolysis |
| "Pyrolysis of Plastics" by ScienceDirect | ScienceDirect Article on Pyrolysis of Plastics |
| "Pyrolysis of Waste Tyres" by Bioresource Technology | Bioresource Technology on Tire Pyrolysis |
| "Pyrolysis of Biomass: A Review" by Renewable and Sustainable Energy Reviews | Review on Pyrolysis of Biomass |
| "Pyrolysis: A Sustainable Way to Manage Organic Waste" by the World Bioenergy Association | World Bioenergy Association - Pyrolysis |
| "Pyrolysis: A Versatile Technology for Converting Biomass to Biofuels and Bioproducts" by the American Chemical Society | ACS Article on Pyrolysis |
| "Pyrolysis of Biomass to Biofuels: A Critical Review" by Energy & Fuels | Critical Review on Pyrolysis of Biomass |
| "Pyrolysis of Waste: A Review" by Waste Management | Waste Management - Pyrolysis Review |
Connect and Network
Please feel free to document your inquiries, observations, or any interest in this topic for research networking or business idea development collaboration.
Proudly powered by TrendyScienceGroup